Structure of an Atom Is Best Described as
Frac11836 This can be described as negligible almost zero. An equal number of protons and neutrons.

Chemical Bonding Atomic Structure And Bonding Britannica
The molecular structure of the IO3- ion is best described as.

. The number of isotopes that are formed by the element. The number of protons in the atomic nucleus. 3The nucleus has a positive charge and is surrounded by negatively charged electrons 4The nucleus has a negative charge and is surrounded by positively charged electrons 4.
Each of these parts has an associated charge. Positive electrons surround a negative nucleus. To write the electron arrangement of an atom follow these steps.
Calculate the inner diameter of the tube in centimeters. 1The nucleus contains positively charged electrons. A neutral atom is best described as having.
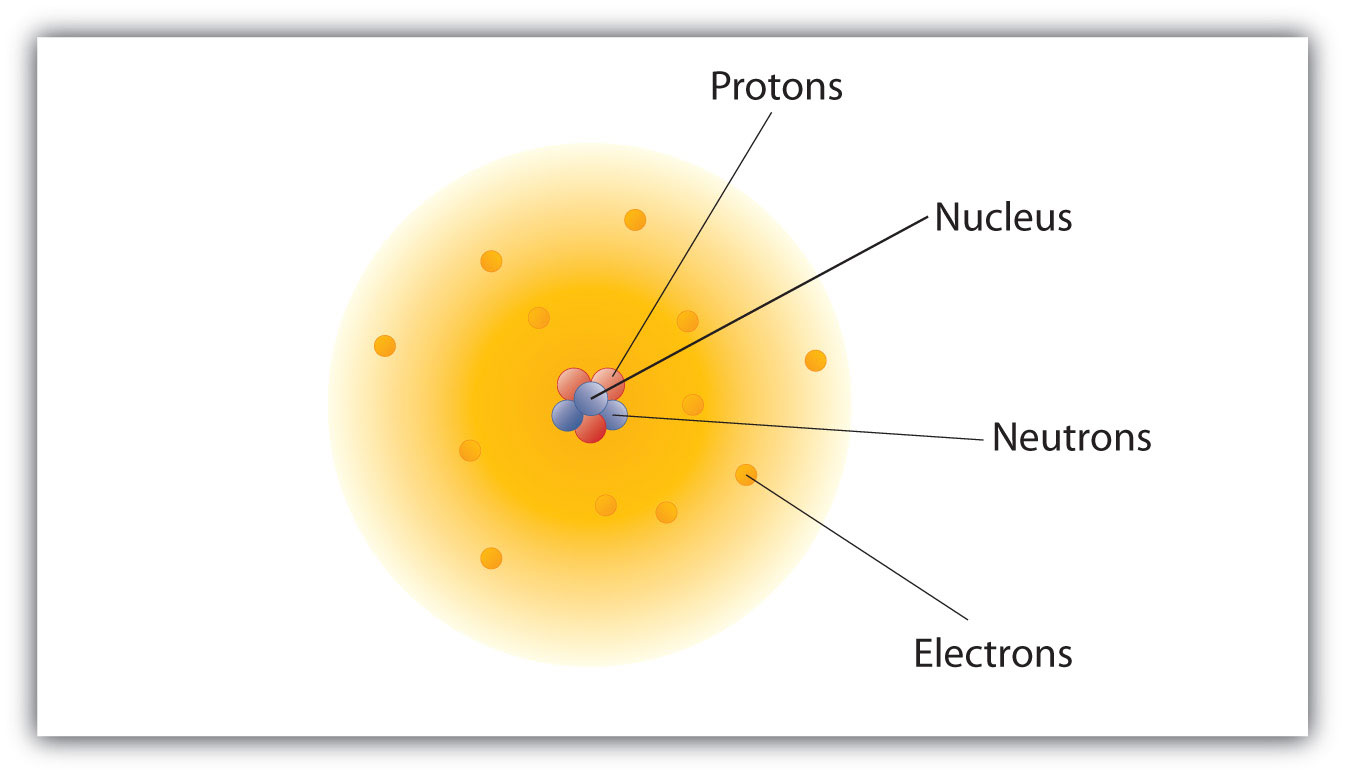
- use atomic mass atomic number and charge to identify neutral atoms ions and isotopes. Atoms consist of a nucleus containing one or more positively charged protons. The number of protons defines an element hydrogen has one proton helium has two and so on of the atom.
The number of charges on ions of the element. An electron in a hydrogen atom moves from level 3 to level 1. From basic chemistry we know that an atom consists of a nucleus that is surrounded by electrons and that the nucleus itself is composed of protons and neutrons.
A 250-cm-long cylindrical glass tube sealed at one end is filled with ethanol. The mass of ethanol needed to fill the tube is found to be 4523 g. On the other hand electrons revolve around the nucleus of the atom.
Which statement about the structure of an atom is correct. Which statement describes the structure of an atom. Stuff You Gotta Know About Atoms.
Electron sharing between two adjacent atoms such that each atom assumes a stable electron configuration both the geometry of and atomic arragements within the unit cell the number of protons and neutrons in the nucleus of an atom as well as the number and. Structure of the atom Nucleus and shells. 2The nucleus contains negatively charged protons.
The innermost shell can hold two electrons. Step 1 Find out the proton number of the atom. The electrons are negatively charged and protons are positively charged while the neutrons are electrically neutral.
All atoms except hydrogen can also contain one or more neutrons in the nucleus. An atom has a central nucleus. Different atoms combine in simle whole-number ratios to form compounds.
More protons than neutrons. More protons than neutrons. Negatively charged electrons orbit the nucleus.
For example a hydrogen atom has one proton in its nucleus and is therefore called number one on the periodic table. Atoms of a specific element are different from those of another elemt. States that matter is composed of extremely small particles called atoms atoms are invisible and indestructable.
Protons and neutrons are placed inside the nucleus of an atom. In a chemcial reaction atoms are separated. - illustrate the structure of the atom by using the Bohr model including the charge relative mass and location of the sub-atomic particles.
The nucleus is tiny compared to the atom as a whole. Structure of the Atom. From Periodic Table Step 2 Find out the number of electrons of the atom.
Negative electrons surround a positive nucleus. Step 3 Arrange the electrons in the shells. For a neutral atom the number of electrons is the same as the number of protons.
An atom of oxygen is smaller than an atom of carbon because the distance of the valence electrons remains the same while the attraction for the nucleus increases with increasing number of protons. In a second hydrogen atom an electron drops from level 2 to level 1. What element an atom is is determined by how many protons it has.
Protons and neutrons make up the nucleus of the atom and electrons orbit the nucleus at different energy levels. An equal number of protons and electrons. 1 The number of electrons in an atom is always the same as the number of protons so atoms are electrically neutral overall.
An atom is made of three parts protons neutrons and electrons. - analyze the structure of the. Atoms of a given element are identical in size mass and chemical properties.
In simple terms an atom is a cloud of tiny electrons buzzing round a central much larger nucleus in a series of orbits called shells. The density of ethanol is 0789 gmL. Atomic structure is best described as.
Thus we can conclude that the structure of an atom is best described as electrons in orbit about a nucleus of protons and neutrons. In a simplified description of the atomic structure based on the. Atomic Structure - describe the characteristics of protons neutrons and electrons in terms of location charge and mass.
A neutral atom is BEST described as having. Positive electrons surround a neutral nucleus. More electrons than protons.
More electrons than protons. The number of protons in the atomic nucleus. This is surrounded by electrons.
Negative electrons surround a neutral nucleus. An equal number of protons and neutrons. The number of protons in a given atoms nucleus is called its atomic number and it is these atomic numbers by which the elements on the periodic table are classified.
Mechanical Engineering questions and answers. The protons carry a positive charge electrons have a negative charge and neutron possess no charge.
The Structure Of The Atom Boundless Chemistry

Atomic Structure Electrons Protons Neutrons And Atomic Models

No comments for "Structure of an Atom Is Best Described as"
Post a Comment